Research Interests
We are interested in delineating the involvement of actin network reorganization in regulating articular cartilage and ocular lens biology and disease. Our vision is to develop novel actin-based therapeutics against disease.
Current Projects
Articular Cartilage
Articular cartilage is the glistening white tissue situated at the end of your joints that provides a nearly frictionless surface to allow for movement of one joint surface over the other. Unfortunately, when damaged, articular cartilage is incapable of self-repair leading to Osteoarthritis. Osteoarthritis is an age-associated, debilitating, chronic disease that cannot be reversed. As the US population continues to age, the proportion of those inflicted with Osteoarthritis is also on the rise. By 2040, one in four individuals will suffer from Osteoarthritis. Our research focuses on understanding how the actin cytoskeleton actively regulates articular cartilage cell (chondrocyte) fate.
Theme 1: Regulation of Chondrocyte Phenotype by Actin
I. Improve current chondrocyte implantation therapies.
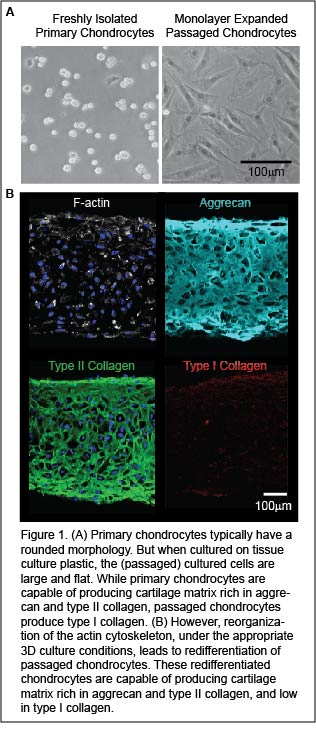
Autologous chondrocyte implantation (ACI) is a state-of-the-art, FDA approved procedure to treat articular cartilage defects. In this procedure small pieces of cartilage (two tic-tacs in size) are harvested from a non-diseased region of a patient’s joint. The cartilage is digested to liberate cartilage cells, or chondrocytes, within the tissue. Chondrocytes are then expanded for cell number by in vitro monolayer passaging. The passaged cells are implanted into damaged cartilage regions to stimulate repair tissue formation. Unfortunately, passaged cells are dedifferentiated. The repair tissue formed by dedifferentiated cells ultimately fails due to the formation of fibrocartilage, which is biomechanically inferior to articular cartilage.
Implicit in chondrocyte dedifferentiation is the reorganization of F-actin from a cortical organization into stress fibers. While treatment of passaged chondrocytes with anti-actin agents (cytochalasins/latrunculins) abolishes stress fibers and represses fibrocartilage formation, these anti-actin treatments are not specific. Not only do they depolymerize actin stress fibers, but they also prevent the formation of cortical actin which is essential for chondrocyte function (i.e. matrix export). In our lab, we are developing new methodologies to abolishes actin stress fibers while maintaining cortical actin in passaged cells. This will lead to the redifferentiation of passaged chondrocytes that have the potential to successfully repair cartilage damage.
II. Examining the role of actin network deregulation during Osteoarthritis.
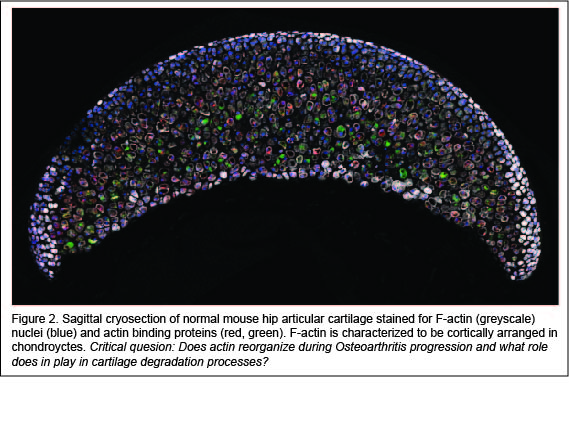
Actin signaling may play a role in Osteoarthritis. Studies show that treatment of chondrocytes with Osteoarthritis inducing cytokines (i.e. interleukin-1, interleukin-6, tumor necrosis factor-α) activates actin polymerization leading to the production of matrix degradative enzymes, such as matrix metalloproteases. However, a major limitation is that previous studies used in vitro isolated chondrocytes cultured in two-dimensions on culture vessels. It is widely accepted that actin organization is context (culture) dependent. To date, there is a very little evidence to show that actin reorganization occurs within the native cartilage environment. Using new imaging high-to-super resolution imaging protocols that we have developed, we are examining actin reorganization in greater-than-before detail, in real time, and in the authentic three-dimensional cartilage tissue architecture. Our projects aim to address the question of how does actin reorganization actively regulate Osteoarthritis?
Theme 2: Ocular Lens
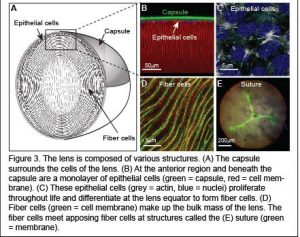
The ocular lens is a transparent tissue situated in the front of the eye that functions to fine focus light onto the retina. The lens must be clear for proper function. However, with age or injury, lens transparency can be lost resulting in cataracts, which are defined as any opacity in the lens that results in cloudy vision. Cataracts is the most common cause of vision loss after the age of forty and the leading cause of blindness world-wide.
Determine ways to target actin reorganization during cataracts.
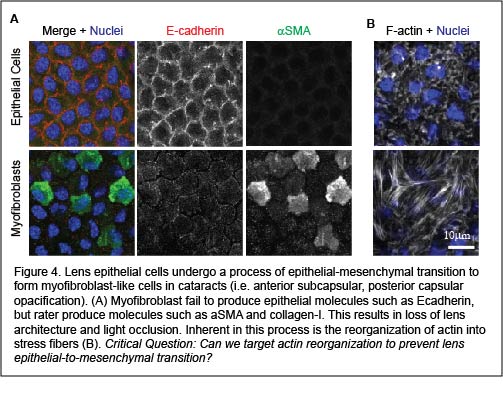
A cause of cataracts is transdifferentiation of the lens epithelial cells (at the anterior region of the lens) into myofibroblasts through the process of epithelial-mesenchymal transition. Early stages of epithelial-mesenchymal transition involve reorganization of cortical actin into stress fibers. While preventing stress fiber formation has been identified as a potential opportunity to prevent epithelial-mesenchymal transition, strategies that specifically target actin stress fiber populations without affecting cortical (epithelial cell) F-actin networks remain elusive. We aim to address the question of what regulates stress fiber formation in lens epithelial cells? And, can we target actin stress fiber formation to prevent cataract formation?
Theme 3: The Contribution of Actin to Tendinosis
Ongoing projects aim to understand the role of F-actin reorganization during tendinosis progression. We are elucidating the role of F-actin in tenocyte mechanotransduction using in vivo, ex vivo, and in vitro approaches. This work is in collaboration with Dr. Dawn Elliott’s group in the Department of Biomedical Engineering.